
Atlantic Canada Poison Centre
Antidote Kit Manual
Acetylcysteine - Single Concentration
Parvolex, Mucomyst, N-Acetylcysteine, NAC
Indications
- Acute acetaminophen overdose (refer to Matthew-Rumack nomogram at end of monograph).
- Repeated supratherapeutic (chronic) acetaminophen ingestion.
- Fulminant hepatic failure due to acetaminophen overdose or other hepatotoxic toxins.
Dosage
A) INTRAVENOUS 21 HOUR PROTOCOL
This protocol will use one standard concentration: Acetylcysteine (NAC) 30 mg/mL. See Administration Section below for preparation instruction.
Dose is calculated using total body weight up to a maximum of 100 kg.
Loading Dose: 150 mg/kg acetylcysteine IV over 60 minutes, immediately followed by:
Continuous Infusion: 15 mg/kg/h acetylcysteine IV for a minimum of 20 h (ALERT — verify rate change on pump)
Infusion Rate Check (patients less than 100 kg) using acetylcysteine 30mg/mL solution:
- Loading Dose; formula: rate (mL/h) = 150 mg/kg x weight (kg) DIVIDED BY 30
- Continuous Infusion; formula: rate (mL/h) = 15 mg/kg/h x weight (kg) DIVIDED BY 30
EXAMPLE for a 60kg patient:
- loading dose is 150 mg/kg x 60 kg divided by 30 mg/mL = 300 mL in 1 h
- continuous infusion is 15 mg/kg/h x 60 kg divided by 30 mg/mL = 30 mL/h
- total volume in 21 hrs = 300 mL + 30 mL/h x 20 h = 900 mL
Infusion Rate Check (patients weighing 100 kg or more; maximum weight for calculations = 100 kg) using acetylcysteine 30mg/mL solution:
- Loading Dose: 15,000 mg (15 g) over 1 hour = 500 mL/h
- Continuous Infusion; 1,500 mg/h = 50 mL/h
B) ORAL 20 HOUR PROTOCOL
Rarely, oral dosing is necessary for patients who develop refractory anaphylactoid reactions to intravenous acetylcysteine. (Refer to Potential Hazards section below)
Loading dose: 140 mg/kg (0.7 mL/kg of 20% acetylcysteine solution). Dilute with soda/juice (2 mL/kg).
Maintenance doses: 70 mg/kg (0.35 mL/kg of 20% acetylcysteine solution) given every 4 hours for five doses. Dilute with soda/juice (1 mL/kg).
| EXAMPLE: ORAL DOSING OF LOADING DOSE FOR A 70KG PERSON |
| Loading Dose: |
| Acetylcysteine 20%: 0.7 mL/kg ( 0.7 mL X 70 kg = 49 mL) |
| Soda/Juice: 2 mL/kg (2 mL X 70 kg = 140 mL) |
| Total Volume to be administered: 49 mL of Acetylcysteine + 140 mL Soda/Juice = 189 mL |
Criteria for Discontinuing NAC (IV or ORAL)
At the end of the 21 h protocol, NAC may be discontinued if all of the following criteria are met: negative acetaminophen level, INR less than or equal to 1.5, AST or ALT less than 50 IU/L OR, if elevated, declining and approximately 50% of the peak value measured. If any of these criteria are not met, continue the NAC at the same rate as the continuous infusion. Bloodwork should then be done every 4 to 12 hours, depending on the clinical scenario. As soon as criteria for stopping NAC is reached, NAC can be discontinued.
Administration
Intravenous 21 hour protocol
Choose bag to prepare according to patient weight. The initial bag will be used for the loading dose and the start of the maintenance infusion.
| Weight | Initial Bag | Additional Bag to complete the 21 h protocol | Subsequent bag to prepare if treatment required beyond 21 h |
| less than 17 kg | 250 mL ** | n/a | 250 mL |
| 17 to 33 kg | 500 mL ** | n/a | 250 mL |
| 34 to 66.6 kg | 1000 mL ** | n/a | 500 mL |
| 66.7 to 99 kg | 1000 mL | 500 mL ** | 500 mL |
| 100 kg or greater | 1000 mL | 500 mL | 500 mL |
** Full volume may not be required to complete the 21 h protocol. To set a total volume for a 21 h protocol, please refer to local pump specific information. Solution remaining can continue to be used if treatment required beyond 21 h.
Prepare bag to a final concentration of 30 mg/mL. Use dextrose 5% in water or sodium chloride 0.9%.
| 250 mL |
1. Remove 37.5 mL from 250 mL bag 2. Add 37.5 mL of NAC 200 mg/mL (20%) to 212.5 mL for a final volume of 250 mL |
|---|---|
| 500 mL |
1. Remove 75 mL from 500 mL bag 2. Add 75 mL of NAC 200 mg/mL (20%) to 425 mL for a final volume of 500 mL |
| 1000 mL |
1. Remove 150 mL from 1000 mL bag 2. Add 150 mL of NAC 200 mg/mL (20%) to 850 mL for a final volume of 1000 mL |
Oral: Calculate and dilute dose according to the “Dosage” section.
Compatibility, Stability
- Stable for 24 hours diluted in dextrose 5% in water or sodium chloride 0.9% at room temperature.
Potential Hazards of Administration
- Nausea, vomiting: can be treated with regular anti-nauseant medications if necessary.
- Anaphylactoid reactions (intravenous dosing). Patients should be monitored closely during the first hour of drug administration. Symptoms include: pruritus, rash, facial edema, urticaria, flushing, chest tightness, tachycardia, hypotension and bronchospasm. Anaphylactoid reactions may be related to the rate of administration and often occur during the loading dose. If anaphylactoid symptoms appear, temporarily discontinue the infusion and assess the patient. Administer an antihistamine if required. If the reaction is severe, follow usual practice for treatment of an allergic reaction. Restart the infusion at a slower rate once the patient is stabilized (i.e., reduce the rate of infusion by half). It is not usually necessary to discontinue acetylcysteine. The entire amount of the acetylcysteine must be administered.
Miscellaneous
- For acute acetaminophen overdose, acetylcysteine is of maximal benefit if initiated within 8 hours post ingestion but is beneficial at greater than 24 hours after ingestion.
- No dosage adjustment required for concomitant dialysis.
- Acetylcysteine 30 mg/mL is slightly hyperosmolar but still within the safety margin for administration via a peripheral vein.
- It is recognized that any particular bag of IV fluids could actually contain fluid in excess of the stated volume, due to overfill. It is of little consequence when making this 30 mg/mL solution. Assume a finished volume as stated on the bag.
Matthew-Rumack Nomogram
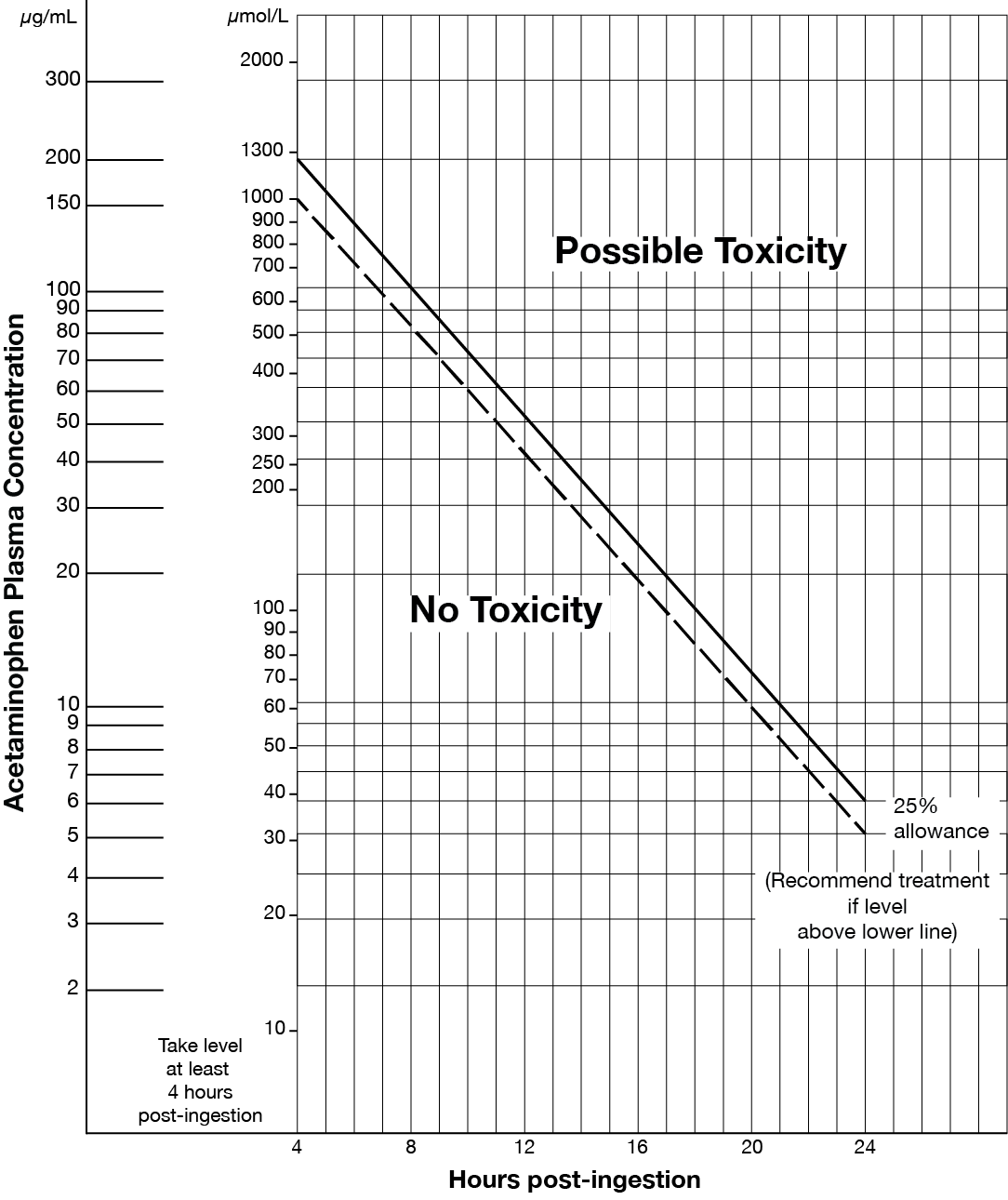
References…
Goldfrank, L. R., Nelson, L. S., Lewin, N. A., Howland, M. A., Hoffman, R. S., (2015). Goldfrank's toxicologic emergencies(Tenth ed.). New York: McGraw Hill.


